TGM Energy: Work, Energy, Heat, and Power
Work
We talk of work as if it's simply the exertion of effort. However, work also has a very precise scientific definition, and consequently very precise units to describe it. The precise unit will be easy to determine once we've given work its definition, so we will waste no further time in doing so. Work is force over distance.
When our car breaks down, and we are forced to push it over to the side of the road, we must do work on it. We can push against the car until we are sweaty and exhausted and collapse onto the road ourselves; but if we have not moved the car, we have done no work. If we have moved the car even an unciaGrafut, however, we've still done quite a bit of work, because the car has a great deal of mass and this motion required considerable force, even though we've moved it only a short distance.
The name "Werg" comes from both "work" and "energy," and this is because on a certain level the two are really the same thing. Work is force over distance; energy is what is required to do a given amount of work. Consequently, the two are measured with the same unit.
TGM's 1:1 correspondence holds here, of course; a Werg is simply one Mag of force over one Grafut of distance. So raising a load of eight Maz to a height of 100 (one biqua) Grafut requires 800 (eight biqua) Wergs of energy. Because of this 1:1 correspondence, we can multiply in this way, greatly simplifying calculations. Neither SI metric nor customary-imperial can boast such simplicity of application.
The SI unit of work and energy is the joule (J), or newton-meter.
Heat
Heat is more than just "that feels hot"; it's the transfer of energy by any means other than work performed on it. Heat should not be confused with temperature, which is simply a way of measuring heat. (There is a more complex and therefore exact scientific definition; but this will do for now.) It is important to remember that heat is simply energy; as such, the TGM unit of heat is the Werg (and the SI unit is the joule).
Traditionally, temperature has been measured in degrees. Celsius, or centigrade, degrees are large; the zero-point of the scale is the freezing point of water, and it is designed to have one hundred (84 in dozenal) degrees between the freezing and boiling points of water at one standard atmosphere of pressure. Fahrenheit degrees are smaller; their zero-point is more or less arbitrary, but serves to keep the vast majority of daily air temperatures positive. It is designed to have one hundred and eighty degrees (130 in dozenal) between the freezing and boiling point of water at a standard atmosphere of pressure. Both of these degree sizes are also used in scientific scales. The Celsius degree is used in the kelvin scale, with the zero point at absolute zero (the coldest possible temperature); this is the scale adopted by SI metric. The Fahrenheit scale is used in the Rankine scale, with the same zero point. Degrees Rankine are rarely used anymore.
Alongside this have been systems which try to keep the specific heat of water equal to one. The specific heat is the amount of energy necessary to raise a given mass of the substance through one unit of temperature increase. For example, customary-imperial commonly employs the "BTU," or "British Thermal Unit"; this is the amount of energy required to raise one pound of water through one degree Fahrenheit. The old calorie was designed to be the amount of heat necessary to raise one gram of water through one degree Celsius; the new calorie, or kilocalorie, is the amount required to raise one kilogram of water through one degree Celsius. There was even a "CHU," or "Centigrade Heat Unit," which was the amount of heat required to raise one pound of water through one degree Celsius. These systems simply did not match up with the systems based on units of energy; one kilocalorie, for example, is about 4.1855 joules (assuming the degree is one kelvin, the same as a degree Celsius; in other words, the specific heat of water in SI metric is 4.1855 J/g*K). SI metric solved this discrepancy by doing away with the whole specific heat of water system entirely; TGM can, and does, do better.
Raising one Maz of water from freezing to boiling required 6E7;7 biquaWerg (2Wg) at a pressure of one Atmoz (2E Prem). Converted into decimal, this means that 1003.6 biquaWerg raises 1 Maz of water through 100.054 degrees Kelvin. Because specific heat varies according to temperature, this gives us 1 biquaWerg raising 1 Maz of water of about 0.1 kelvin on average; this further means that 1 Werg raises 1 Maz of water through 0;001249 K. This provides our unit of temperature, the Calg:
The Calg is a scientific unit intended to be used on a scientific scale; therefore, its zero point is absolute zero, like the SI metric kelvin scale and the Rankine scale. For daily use, more convenient would be the biquaCalg or the triquaCalg, which can be called, for convenience's sake, the decigree and the tregree respectively. Experience has shown that tregrees, symbol "t°," tend to be most useful.
 |
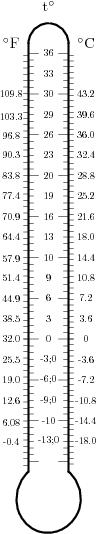 |
The scales above illustrate the Calg in actual use. To the left is a scale comparing quadquaCalgs to kilorankines and kilokelvins; this gives a scale from very cold to very hot, and shows the relations of the three systems quite nicely. (The boiling point of table salt, NaCl, is simply included for curiosity's sake.) The scale on the right shows tregrees compared to degrees Celsius and degree Fahrenheit at normal, daily scales. It clearly shows that tregrees, although sized as triquaCalgs, are considered with a zero point at the freezing point of water.
Power
Power is work per unit time, or in other words the rate at which work is performed. In the metric system, the unit of power is the watt (W); in customary-imperial, there are a variety of units used for power, most particularly the erg per second, the horsepower, and the foot-pound per minute. (There are also more creative constructions, like the BTU/hr.) In TGM, the unit of power is the Pov:
By chance, the conversion factor to the watt is quite close to 300 (three biqua); this makes converting between TGM and SI metric in power units quite easy. For example, one 2Pv (one biciaPov) is less than a thousandth less than three watts, so 1 2Pv = 3 W is accurate enough for most purposes.